Science
New Supernova Stent Shows Promise for Stroke Treatment in India
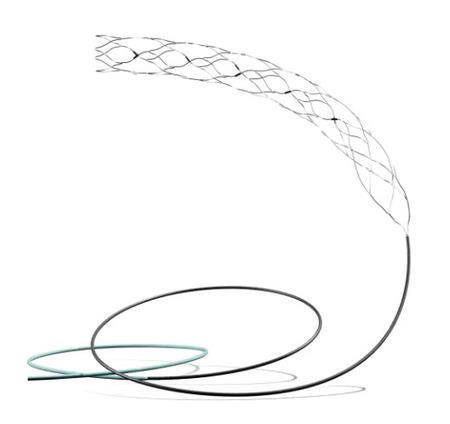
A recent clinical trial led by experts at the All India Institute of Medical Sciences (AIIMS) in New Delhi has highlighted the potential of a new device called the Supernova Stent for treating stroke patients. According to findings from the GRASSROOT trial, this advanced brain treatment device has demonstrated both safety and effectiveness in addressing severe strokes.
Dr. Shailesh B. Gaikwad, Professor and Head of the Department of Neuroimaging & Interventional Neuroradiology at AIIMS, serves as the National Principal Investigator for the trial. He described the study as a significant turning point for stroke treatment in India. The preliminary results, published in the esteemed Journal of Neurointerventional Surgery (JNIS), indicate that the Supernova stent has achieved excellent outcomes in terms of safety and efficacy.
The GRASSROOT trial marks the first prospective multicentre thrombectomy trial, focusing on the physical removal of blood clots from blocked arteries. The Supernova stent retriever successfully restored blood flow in a high percentage of cases, with a reported brain bleed rate of 3.1 percent and a mortality rate of 9.4 percent. Remarkably, the trial also noted that 50 percent of patients achieved functional independence within 90 days following treatment.
Developed by Gravity Medical Technology, the Supernova stent is tailored to meet the needs of India’s diverse population, where strokes tend to affect younger individuals compared to Western countries. Earlier this year, data from the GRASSROOT trial received approval from the Central Drugs Standard Control Organisation (CDSCO), paving the way for the stent’s routine use in India.
This major clinical trial was conducted across eight centres, affirming the device’s safety and efficacy in tackling life-threatening strokes. Experts believe that it represents a significant milestone for the Make-in-India initiative, positioning the country as a global player in advanced stroke care.
Dr. Dileep Yavagal, a Professor of Neurology and Neurosurgery at the University of Miami, who participated in the global trial, noted that the device has already been used to treat over 300 patients in Southeast Asia. The Supernova stent will now be manufactured in India and made available at affordable prices, providing new hope to the estimated 1.7 million Indians who suffer strokes each year.
The promising results of the GRASSROOT trial not only highlight advancements in medical technology but also underscore the importance of innovative solutions in addressing critical health challenges. As the Supernova stent becomes more widely available, it is anticipated to significantly impact stroke treatment in India and beyond.
-

 Business5 months ago
Business5 months agoKenvue Dismisses CEO Thibaut Mongon as Strategic Review Advances
-

 Lifestyle5 months ago
Lifestyle5 months agoHumanism Camp Engages 250 Youths in Summer Fest 2025
-

 Sports5 months ago
Sports5 months agoDe Minaur Triumphs at Washington Open After Thrilling Comeback
-

 Sports5 months ago
Sports5 months agoTupou and Daugunu Join First Nations Squad for Lions Clash
-

 Top Stories5 months ago
Top Stories5 months agoColombian Senator Miguel Uribe Shows Signs of Recovery After Attack
-

 World5 months ago
World5 months agoASEAN Gears Up for Historic Joint Meeting of Foreign and Economic Ministers
-

 Health5 months ago
Health5 months agoNew Study Challenges Assumptions About Aging and Inflammation
-

 Business5 months ago
Business5 months agoOil Prices Surge Following New EU Sanctions on Russia
-

 World3 months ago
World3 months agoSouth Korea’s Foreign Minister Cho Hyun to Visit China This Week
-

 Entertainment5 months ago
Entertainment5 months agoDetaşe-Sabah Violin Ensemble Captivates at Gabala Music Festival
-

 Business3 months ago
Business3 months agoStarling Bank Plans Secondary Share Sale, Targeting $5.4 Billion Valuation
-

 Entertainment5 months ago
Entertainment5 months agoBaku Metro Extends Hours for Justin Timberlake Concert









