Science
Advanced Supernova Stent Shows Promise for Stroke Treatment
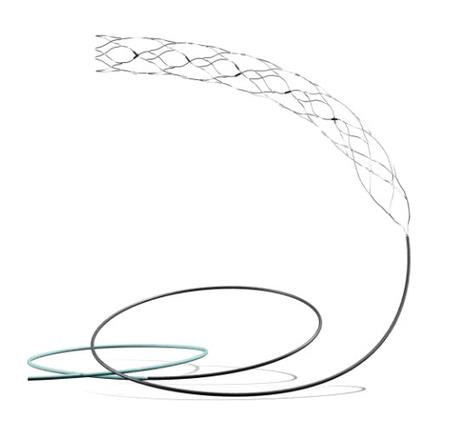
In a groundbreaking development for stroke treatment, the Supernova Stent has been shown to be both safe and effective for patients suffering from severe strokes, according to results from a clinical trial led by the All India Institute of Medical Sciences (AIIMS) in Delhi. This trial, known as the GRASSROOT Trial, marks a significant milestone in advanced stroke care in India.
On December 13, 2023, experts from AIIMS announced that the Supernova Stent demonstrated promising results, indicating it could transform the treatment landscape for stroke patients in the country. Dr. Shailesh B. Gaikwad, Professor and Head of the Department of Neuroimaging & Interventional Neuroradiology at AIIMS and the National Principal Investigator of the GRASSROOT Trial, stated, “This trial is a turning point for stroke treatment in India.” He noted that preliminary findings published in the Journal of Neurointerventional Surgery revealed excellent safety and efficacy outcomes.
Breakthrough Results in Stroke Care
The GRASSROOT Trial is the first prospective multicentre thrombectomy trial to utilize the Supernova stent retriever, which has achieved a high success rate in restoring blood flow to the brain. The trial reported a brain bleed rate of just 3.1 percent and a mortality rate of 9.4 percent. Additionally, the results showed that 50 percent of participants achieved functional independence within 90 days post-procedure.
Designed specifically for the diverse patient population in India, the Supernova Stent addresses a pressing need. Strokes in India often affect younger individuals compared to Western countries. Earlier this year, the Central Drugs Standard Control Organisation (CDSCO) accepted data from the GRASSROOT trial, leading to the stent’s approval for routine use in India.
A Step Forward for Make-in-India Initiative
The GRASSROOT Trial was conducted across eight medical centers, highlighting India’s growing capabilities in advanced medical technology. The success of this trial not only supports the Make-in-India initiative but also positions India as a key player in the global landscape of stroke treatment.
Dr. Dileep Yavagal, a Professor of Neurology and Neurosurgery at the University of Miami and part of the global trial, shared insights on the broader implications of the Supernova Stent. “The device has already treated more than 300 patients in Southeast Asia and is now set to be manufactured in India at affordable prices,” he stated. This development offers new hope to the approximately 1.7 million Indians who experience strokes annually.
The advancements from the GRASSROOT Trial represent not only a medical achievement but also a significant step toward improving healthcare accessibility and outcomes for stroke patients in India and beyond.
-

 Business5 months ago
Business5 months agoKenvue Dismisses CEO Thibaut Mongon as Strategic Review Advances
-

 Lifestyle5 months ago
Lifestyle5 months agoHumanism Camp Engages 250 Youths in Summer Fest 2025
-

 Sports5 months ago
Sports5 months agoDe Minaur Triumphs at Washington Open After Thrilling Comeback
-

 Sports5 months ago
Sports5 months agoTupou and Daugunu Join First Nations Squad for Lions Clash
-

 Top Stories5 months ago
Top Stories5 months agoColombian Senator Miguel Uribe Shows Signs of Recovery After Attack
-

 World5 months ago
World5 months agoASEAN Gears Up for Historic Joint Meeting of Foreign and Economic Ministers
-

 Health5 months ago
Health5 months agoNew Study Challenges Assumptions About Aging and Inflammation
-

 Business5 months ago
Business5 months agoOil Prices Surge Following New EU Sanctions on Russia
-

 World3 months ago
World3 months agoSouth Korea’s Foreign Minister Cho Hyun to Visit China This Week
-

 Entertainment5 months ago
Entertainment5 months agoDetaşe-Sabah Violin Ensemble Captivates at Gabala Music Festival
-

 Business3 months ago
Business3 months agoStarling Bank Plans Secondary Share Sale, Targeting $5.4 Billion Valuation
-

 Entertainment5 months ago
Entertainment5 months agoBaku Metro Extends Hours for Justin Timberlake Concert









