Health
Ark Biopharmaceutical Gains Approval for ADHD Treatment in China
Shanghai Ark Biopharmaceutical Co., Ltd. has received an important endorsement from China’s National Medical Products Administration (NMPA) for its product, Aizhida, designed to treat Attention Deficit Hyperactivity Disorder (ADHD) in patients aged six years and older. This approval marks a significant advancement in ADHD treatment options within China, where the prevalence of the disorder among children and adolescents is estimated to be around 6.4%, impacting over 23 million individuals.
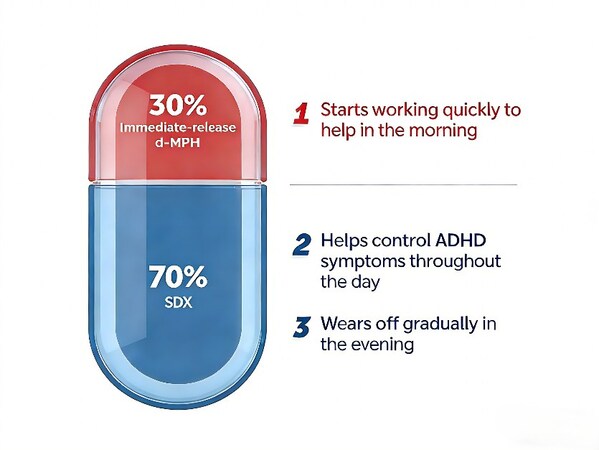
Aizhida comprises two active ingredients: serdexmethylphenidate chloride and dexmethylphenidate hydrochloride. This combination medication operates as a central nervous system stimulant, formulated to provide both rapid relief and extended symptom management. The once-daily capsule features an immediate-release component that acts quickly, while the prodrug serdexmethylphenidate is gradually converted to dexmethylphenidate in the lower gastrointestinal tract, promoting sustained therapeutic effects throughout the day.
Addressing Clinical Needs in China
The approval of Aizhida is particularly crucial given the limited treatment options currently available for ADHD in China. Existing medications often fail to meet the full clinical requirements of patients, leading some to discontinue their treatments due to inadequate response, side effects, or difficulties in dosing. The introduction of Aizhida aims to fill this gap by offering a novel therapeutic alternative.
In a pivotal Phase III clinical trial involving Chinese patients, Aizhida demonstrated statistically significant improvements in core ADHD symptoms compared to a placebo, achieving both primary and key secondary endpoints. This data underscores the efficacy of Aizhida, providing a much-needed option for clinicians and patients grappling with ADHD.
Professor Yi Zheng, Chief Expert at Beijing Anding Hospital and Lead Principal Investigator of the Aizhida trial, emphasized the importance of this development: “ADHD is one of the most common neurodevelopmental disorders in childhood, with potential impacts lasting into adulthood. The approval of Aizhida provides clinicians with a new therapeutic tool that can help improve the treatment landscape for ADHD patients in China.”
Innovative Treatment Paradigm
Aizhida’s unique dual mechanism of action, combining immediate-release and prodrug-based extended-release technologies, positions it to potentially transform the ADHD treatment paradigm in the country. Its profile of “rapid onset + full-day coverage” is set to redefine how ADHD is managed, offering patients an effective and convenient option.
Professor Jing Liu, Director of the Child Psychiatry Center at Peking University Sixth Hospital and another Lead Principal Investigator for the trial, highlighted the broader implications of Aizhida’s approval: “We are committed to advancing standardized diagnosis and treatment for pediatric mental disorders in China. The approval of a new drug not only increases options but also prompts deeper reflection on optimizing treatment strategies.”
With Aizhida now entering the commercial launch phase, Ark Biopharmaceutical is poised to make a significant impact on the ADHD treatment landscape in China. The company’s commitment to developing innovative therapeutics is underscored by its established collaborations with prominent pharmaceutical companies and academic institutions, including Roche and the Scripps Research Institute.
As the healthcare community in China anticipates the real-world application of Aizhida, there is hope that this new treatment will enhance the standard of care for patients suffering from ADHD, improving their long-term outcomes and overall quality of life.
For more information about Ark Biopharmaceutical and its innovative products, visit their official website at www.arkbiosciences.com.
-

 World5 months ago
World5 months agoSouth Korea’s Foreign Minister Cho Hyun to Visit China This Week
-

 Business5 months ago
Business5 months agoStarling Bank Plans Secondary Share Sale, Targeting $5.4 Billion Valuation
-

 Top Stories5 months ago
Top Stories5 months agoMunsang College Celebrates 100 Years with Grand Ceremony
-

 World5 months ago
World5 months agoPAS Aims to Expand Parliamentary Influence in Upcoming Election
-

 Business7 months ago
Business7 months agoKenvue Dismisses CEO Thibaut Mongon as Strategic Review Advances
-

 Lifestyle6 months ago
Lifestyle6 months agoHumanism Camp Engages 250 Youths in Summer Fest 2025
-

 Sports6 months ago
Sports6 months agoDe Minaur Triumphs at Washington Open After Thrilling Comeback
-

 Sports7 months ago
Sports7 months agoTupou and Daugunu Join First Nations Squad for Lions Clash
-

 Top Stories7 months ago
Top Stories7 months agoColombian Senator Miguel Uribe Shows Signs of Recovery After Attack
-

 World7 months ago
World7 months agoASEAN Gears Up for Historic Joint Meeting of Foreign and Economic Ministers
-

 Health6 months ago
Health6 months agoNew Study Challenges Assumptions About Aging and Inflammation
-

 Business7 months ago
Business7 months agoOil Prices Surge Following New EU Sanctions on Russia









